Apr 16, 2025
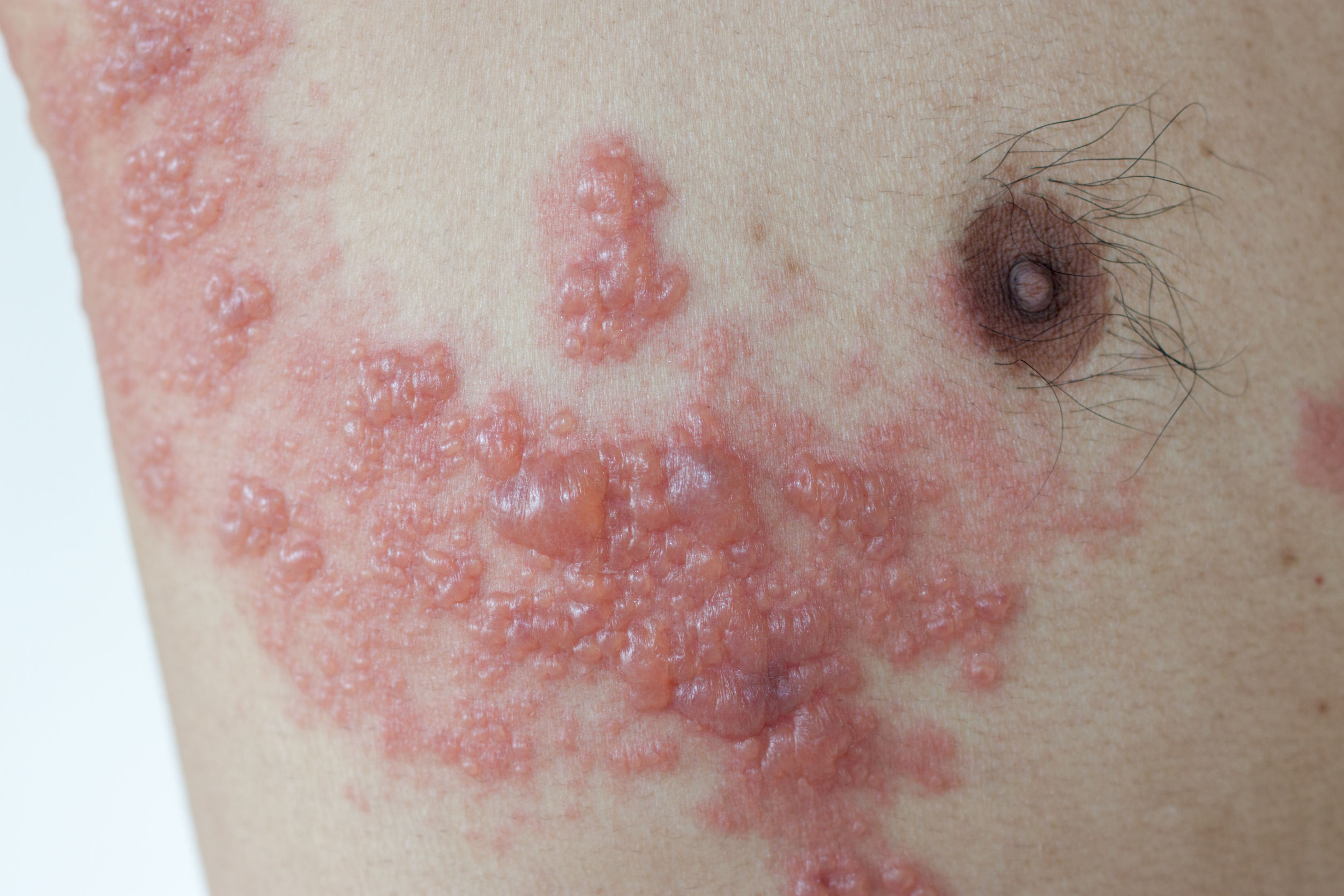
Studies show that the shingles vaccine reduces the risk of developing shingles by approximately 51% in individuals aged 60+. It also reduces the severity and post-infection pain (postherpetic neuralgia) by about 67%.
Reduces shingles risk by ~51% in those aged 60+.
Reduces severity and post-shingles pain by ~67%.
Adults >60 years old, regardless of prior chickenpox history.
Adults aged 50–59 years with a history of recurrent shingles or prior chickenpox.
Single dose administered subcutaneously (under the skin).
Allergy to vaccine components (e.g., gelatin, neomycin).
Severe immunodeficiency.
Acute illness (e.g., high fever, active infection)—delay vaccination until recovery.
Pregnancy.